Introduction
In the movie GATTACA, Ethan Hawke’s parents visit a doctor’s office to choose the embryo of their second child. They have a choice between four babies—two boys, two girls—each of which has been engineered to perfection. As the doctor says: “[These embryos have] no predispositions to any major inheritable diseases. All that remains is to select the most compatible candidate.”
 Screenshot from GATTACA
Screenshot from GATTACA
GATTACA was produced in 1997. Although we can’t yet choose our child’s intelligence by modifying its embryo, gene editing has come a long way since. We have sequenced the entire human genome. We have discovered how to write biological material like software.
I expect biological engineering to be one of the most important technological advances in my lifetime, so I wanted to learn how it works in practice.
Fortunately for me, New York City has a community biolab called Genspace. Community biolabs are labs at which ordinary citizens can learn about and work on biotechnology. After taking an introductory course in biotechnology in November 2017, I signed up for a course in gene editing this February. The technique we would use is called CRISPR-Cas9.
What is CRISPR–Cas9?
CRISPR-Cas9 is a gene editing technology inspired by nature. CRISPRs—short strips of genetic code—are part of a defense method that bacteria have developed to protect themselves from intruding viruses. When a virus intrudes into a bacterial cell, a protein inside the bacterial cell can identify the virus (using CRISPRs) and cut it in two, disabling the virus.
In 2012, scientists at UC Berkeley first successfully modified this method to target a custom, programmed gene sequence. In 5 years since, CRISPR has proliferated, so much so that you can buy do-it-yourself CRISPR kits online.
The first two minutes of this video by MIT explain the mechanism well.
What did I do in my course?
The goal of the course was to modify yeast’s DNA. Yeast naturally looks white; we had to color it red and make it green fluorescent under ultraviolet light.
This metamorphosis consisted of two steps. First, we had to disable the gene that made the yeast look white (if this gene was not expressed, the yeast would look red instead). Second, we had to add new DNA to the yeast that would make it green fluorescent. CRISPR-Cas9 can do both—disable existing genes by cutting them and inserting new genes by adding new nucleotides.
To do this, I must have been a biology expert, right? Far from it. I took 3 years of high school bio and never did serious lab work. I did not take any biology after the age of 14—I could barely remember that animal cells have a nucleus and bacterial cells don’t. Now I was going to modify the DNA of a living organism? Yes.
So how would I turn our standard white yeast into a red-and-green, disco-loving creature?
White to Red
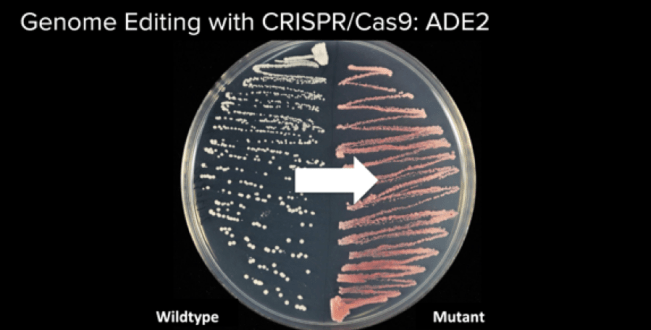 Our goal was to turn yeast from its natural white color (left) to red (right). Photo courtesy of Will Shindel, Genspace’s instructor.
Our goal was to turn yeast from its natural white color (left) to red (right). Photo courtesy of Will Shindel, Genspace’s instructor.
We had to cut the gene that turns the yeast white. The cutting of DNA is done by the Cas9 protein. This protein must be instructed to go after the right target—in this case, the yeast’s gene that gives it its white color. To instruct the Cas9 protein (think of this as the scissors) we had to add a piece of guide RNA. Guide RNA is like a barcode: it tells the Cas9 protein what sequence of DNA to look for, and once it finds it, the Cas9 protein will make the cut. So, in addition to the Cas9 protein (the scissors), we had to add guide RNA (the barcode).
Minutes 1:50 to 2:50 of the same MIT video explain this process of cutting existing genes using Cas9 proteins (scissors) tailored with guide RNA (barcodes).
Using CRISPR-Cas9, you can now modify this process to go after any genetic code you like by modifying the barcode.
Amazon meets Biotech
 Screenshot of CRISPRdirect, a website that helps you to identify the guide RNA sequence with the highest likelihood of cutting your gene successfully.
Screenshot of CRISPRdirect, a website that helps you to identify the guide RNA sequence with the highest likelihood of cutting your gene successfully.
How do you create the barcode? This part is fascinating. You simply search on Google for the gene of the organism you want to modify—in our case, the gene in yeast that gives it its white color. (The gene is called ADE2). You then download the gene’s DNA code—a series of letters A, T, C, and G—and paste it into Snapgene, a simple program that helps you read the DNA more easily. You then past the DNA code into CRISPRdirect, to identify the guide RNA (the barcode) with the highest likelihood of cutting the DNA properly, and then order the guide RNA (the barcode) using a website like IDT. At that instant, machines will start whizzing hundreds of miles away to synthesize the genetic code that you just submitted through a form on a website. The RNA is delivered to your doorstep within 24 hours.
Wow.
Introducing the scissors and the barcode was enough to disable the yeast’s red color. But changing white to red was only half our goal.
Special Effects, Please
The second step was to make the yeast glow bright green. To do this, we had to add extra genetic materials.
After the Cas9 protein (scissors) cut the yeast’s DNA at the site instructed by the guide RNA (barcode), the yeast’s DNA two strands (double helix, remember?) will naturally try to heal. This is when you can introduce new genetic material into the DNA.
The trick is to hide the new, foreign DNA in other genetic materials that the yeast recognizes as naturally occurring. It’s a bit like hiding your dog’s medicine inside his food, hoping that he will eat the medicine unknowingly by finishing his food.
We wanted to add new material to code for our green fluorescent gene. This material was a series of 1510 nucleotides. (Nucleotides are single molecules that humans codify by the letters A, C, G, or T, which stands for the molecules Adenine, Cytosine, Guanine, and Thymine.) To both ends of the new gene that would lead to green fluorescence, we added DNA that matched both sides of the cut in yeast’s DNA. Normally, after a cut, the two strands of yeast DNA will naturally heal back in their own place, as explained in the previous video. However, when enough “repair material” is present in the yeast’s cell, the yeast’s DNA will heal instead by connecting with this new repair material. As a consequence, you have now successfully inserted a little bit of new DNA into the yeast’s original code.
 The blue, double strand is the original DNA; the purple, single strand of DNA is the inserted genetic material. This screenshot shows how the purple, new DNA is being connected with the outermost 4 base pairs onto the blue, original DNA.
The blue, double strand is the original DNA; the purple, single strand of DNA is the inserted genetic material. This screenshot shows how the purple, new DNA is being connected with the outermost 4 base pairs onto the blue, original DNA.
In my mind, I compare this mechanism to how magnets work. The naturally recognized pieces of DNA that you add to both sides of the newly introduced gene (green fluorescent, in our case) are like magnets. These magnets were chosen to have a strong attraction to both sides of the cut of the original DNA. When the green fluorescent gene with magnets on both sides comes close to the original DNA cut by CRISPR-Cas9, the magnets on the side of the green fluorescent gene connect with both sides of the original DNA’s cut, and as a result, the updated yeast’s DNA now has a line of code that makes it green fluorescent.
Minutes 2:50 onward of the same MIT video explain the process of inserting new genetic materials into existing genes well.
Results
A week after we added the cutting sequence (to cut the yeast’s ADE2 gene that made it look white) and the DNA that would make the yeast green fluorescent to the yeast, we returned to the lab to look at our results.
Our yeast colonies had replicated, and most samples showed red yeast instead of white yeast. Unfortunately, none of the colonies turned green fluorescent under UV light (despite what the image below seems to show—if the green fluorescent gene would have been adopted, the yeast colonies would have shown much greener).
 Petri dishes with yeast colonies under UV light. The petri dish top-center and bottom-right show mold growth (this is contamination).
Petri dishes with yeast colonies under UV light. The petri dish top-center and bottom-right show mold growth (this is contamination).
A possible reason why the green fluorescent gene was not integrated was that the ADE2 genes were indeed broken (hence the shift from white to red), but that they reconnected with a different sequence, and that therefore the green fluorescent protein (GFP) was not adopted.
Accessible Science
Now that I have edited live genes, what are my reflections?
It’s hard to believe that somebody with no deep background in biology can understand and learn how to edit DNA in less than a day. I don’t suggest that I have mastered the discipline—far from it, of course—but I have learned how to practice the basics.
The technologies used to modify DNA are relatively simple too. The tools used are relatively simple—either pipettes, to dose liquids, or devices that spin, heat, or cool the genetic material.
Will We Edit Human Embryos Soon?
Chinese researchers have started to edit human embryos. And last summer, the United States followed suit, with the first American editing of a human embryo (using CRISPR-Cas9), see the video below. Like in GATTACA, will all babies soon be edited?

In the short term, I don’t think so. Our understanding of the human genome is too limited. We know how some genes code for some features, but we are far away from knowing what genes make you smart, tall, or strong. 23 and me, a service that synthesizes your DNA for $199, will tell you if your urine will smell after you eat asparagus, or if you’re likely to grow bald, but it will not tell you your IQ or whether you’re good at public speaking.
Our understanding of human genes is expanding rapidly though. Almost every issue of New Scientist reports on the discovery of a new gene. Stephen Hsu, VP of Research at Michigan State University, recently led a study that can predict the height a person will be within a 3 centimeter range based on the person’s DNA.
So as we learn more about genes, will we allow “editing” of humans?
I think the answer is yes. If you are the parent of a child that will be born with a terrible, hereditary disease, and it is possible to save your child from that suffering, would you not?
Even if some countries don’t allow it, others will. Wealthy people who care about giving their children the best possible genes will go to the countries that allow for gene editing and use the technique to modify their embryos.
Initially this will be done only to disable hereditary diseases. But what about modifying genes to upgrade ourselves: making our children more intelligent, better-looking, or stronger?
That will happen too. Practices will spring up in which the ultra-wealthy can “upgrade” their embryos. Some people will choose to do this, because the surest way to feel you’re leaving something for future generations is to improve the chances of your offspring being successful.
If you’re in that position, what would you do?
Gaaf!
Verstuurd vanaf mijn iPhone
>
heel interessant maar ook beangstigend. “Perfectionisme” bestaat alleen in de geest, niet in de natuur. Juist “afwijkingen” blijken vaak veel te brengen. Doordat we iets niet goed kunnen activeren we andere delen van ons brein en persoonlijkheid. Ieder mens heeft zijn beperkingen. We hebben te leren hoe we deze beperkingen kunnen omhelzen in plaats van ze te ontkennen. Daar schuilt de schoonheid en kracht in van het leven. Als iemand gehandicapt is, krijgt een andere mens de kans om liefde en compassie te tonen. Daardoor wordt de mens een superman of woman, niet door DNA modifacite
modificatie, had daar moeten staan.